The Tuskegee Syphilis Experiment
Introduction
The Tuskegee Syphilis Experiment is one of the most infamous cases of unethical medical experimentation in American history. Conducted over a span of 40 years, this study not only violated the trust of its participants but also highlighted the deep-seated racial prejudices that existed within the medical community. Imagine being promised free healthcare, only to discover that you were part of a cruel experiment where your suffering was observed rather than treated. This article delves into the details of the Tuskegee Syphilis Study, exploring its history, ethical violations, and lasting impact on public health and trust in medical institutions.
What Was the Tuskegee Syphilis Experiment?
Overview of the Study
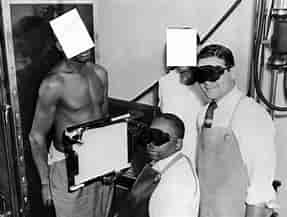
Officially known as the “Tuskegee Study of Untreated Syphilis in the Negro Male,” this study began in 1932 and continued until 1972. It was conducted by the U.S. Public Health Service (PHS) in collaboration with the Tuskegee Institute in Alabama. The primary goal was to observe the natural progression of untreated syphilis in African American men.
Recruitment of Participants
The study involved 600 African American men from Macon County, Alabama. Of these, 399 had latent syphilis, while 201 served as a control group without the disease. Participants were mostly impoverished sharecroppers who were lured into the study with promises of free medical care, meals, and burial insurance.
The Deception Behind the Study
Misleading Information
Participants were told they were being treated for “bad blood,” a local term that encompassed various ailments, including anemia and fatigue. They were never informed about their syphilis diagnosis or the nature of the study itself.
Withholding Treatment
As medical advancements made syphilis treatable with penicillin by the late 1940s, researchers deliberately withheld this effective treatment from participants. Instead, they continued to observe their health decline without intervention.
Ethical Violations in Medical Research
Lack of Informed Consent
One of the most glaring ethical violations in the Tuskegee Study was the absence of informed consent. Participants were not adequately informed about their condition or the risks involved in being part of this study. This lack of transparency is a fundamental breach of ethical research practices.
Exploitation of Vulnerable Populations
The study exploited a marginalized group—poor African American men—who had limited access to healthcare and education. This exploitation raises serious questions about racial discrimination and ethical responsibilities in medical research.
The Impact on Participants
Health Consequences
Many participants suffered severe health consequences due to untreated syphilis. The disease can lead to serious complications, including cardiovascular damage, neurological issues, and even death. It is estimated that over 100 men died as a direct result of their untreated syphilis.

Psychological Effects
Beyond physical health issues, participants experienced significant psychological distress. The betrayal by medical professionals they trusted likely contributed to feelings of hopelessness and despair.
Public Outcry and Exposure
Whistleblower Revelations
In the mid-1960s, Peter Buxtun, a PHS employee, became increasingly concerned about the ethical implications of the study. He attempted to alert authorities about its unethical nature but faced resistance.
Media Coverage
The turning point came in 1972 when an Associated Press article exposed the study’s unethical practices to the public. This revelation led to widespread outrage and demands for accountability.
Legal Repercussions and Settlements
Class-Action Lawsuit
Following public outcry, a class-action lawsuit was filed on behalf of the participants and their families. In 1974, it resulted in a $10 million settlement from the federal government.
Establishment of Guidelines for Ethical Research
The Tuskegee Study prompted significant changes in how medical research is conducted in the United States. It led to the establishment of regulations requiring informed consent and ethical oversight for studies involving human subjects.
The Legacy of the Tuskegee Syphilis Experiment
Impact on Public Trust
The Tuskegee Study has had a lasting impact on public trust in medical institutions, particularly within African American communities. This betrayal has created skepticism towards healthcare providers and research initiatives.
Influence on Medical Ethics Education
The study serves as a critical case study in medical ethics education programs across universities and institutions. It highlights the importance of ethical considerations in research practices and patient care.
Lessons Learned from Tuskegee
Importance of Informed Consent
One crucial lesson from this dark chapter in history is the necessity for informed consent in all medical research. Participants must be fully aware of their rights and understand any potential risks involved.
Ethical Oversight is Essential
Establishing independent review boards to oversee research involving human subjects is vital to prevent ethical violations similar to those seen during the Tuskegee Study.

Moving Forward: Building Trust in Healthcare
Engaging Communities
To rebuild trust within marginalized communities, healthcare providers must actively engage with these populations through education and outreach programs that address historical injustices.
Promoting Transparency
Transparency about research practices and treatment options can help mitigate fears stemming from past abuses like those seen in Tuskegee. Open communication fosters trust between patients and providers.
Conclusion: Remembering History to Shape a Better Future
The Tuskegee Syphilis Experiment serves as a stark reminder of how far we have come—and how far we still need to go—in ensuring ethical practices within medicine and research. By understanding this painful chapter in history, we can work toward building a more equitable healthcare system that respects all individuals’ rights and dignity.
As we reflect on these lessons learned from Tuskegee, let’s commit ourselves to ensuring that such unethical practices are never repeated. Together, we can advocate for transparency, informed consent, and respect for all individuals—because everyone deserves dignity in healthcare.
FAQs
1. What was the purpose of the Tuskegee Syphilis Experiment?
The purpose was to observe untreated syphilis’s natural progression among African American men without providing them with effective treatment or informing them about their diagnosis.
2. How long did the Tuskegee Study last?
The study lasted for 40 years, from 1932 until it was exposed publicly in 1972.
3. What were some key ethical violations during this experiment?
Key violations included lack of informed consent, deception regarding participants’ health status, and withholding effective treatment despite its availability.
4. What impact did this study have on public trust?
It significantly eroded trust between African American communities and healthcare institutions due to its exploitative nature and racial discrimination.
5. How did society respond after learning about this experiment?
After exposure, there was widespread outrage leading to legal action against the government; it also prompted changes in regulations governing human subjects’ research ethics.